Lorlatinib | Lorbrena
Jump to a section
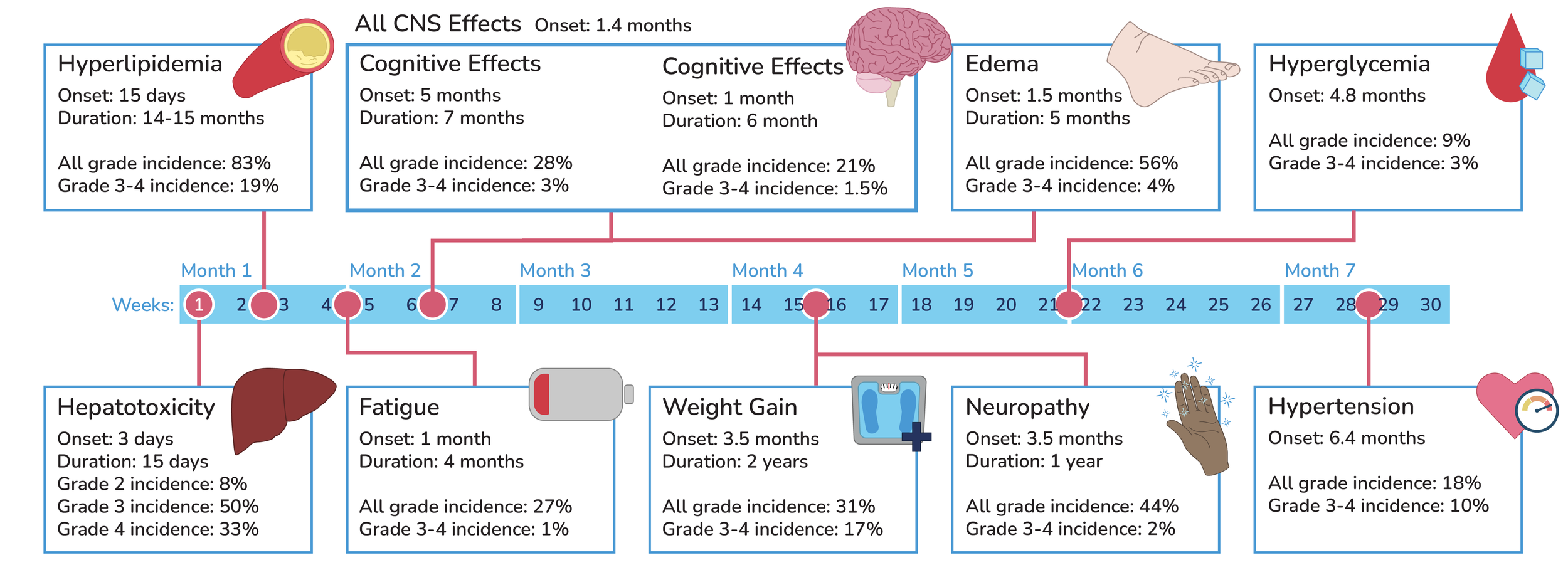
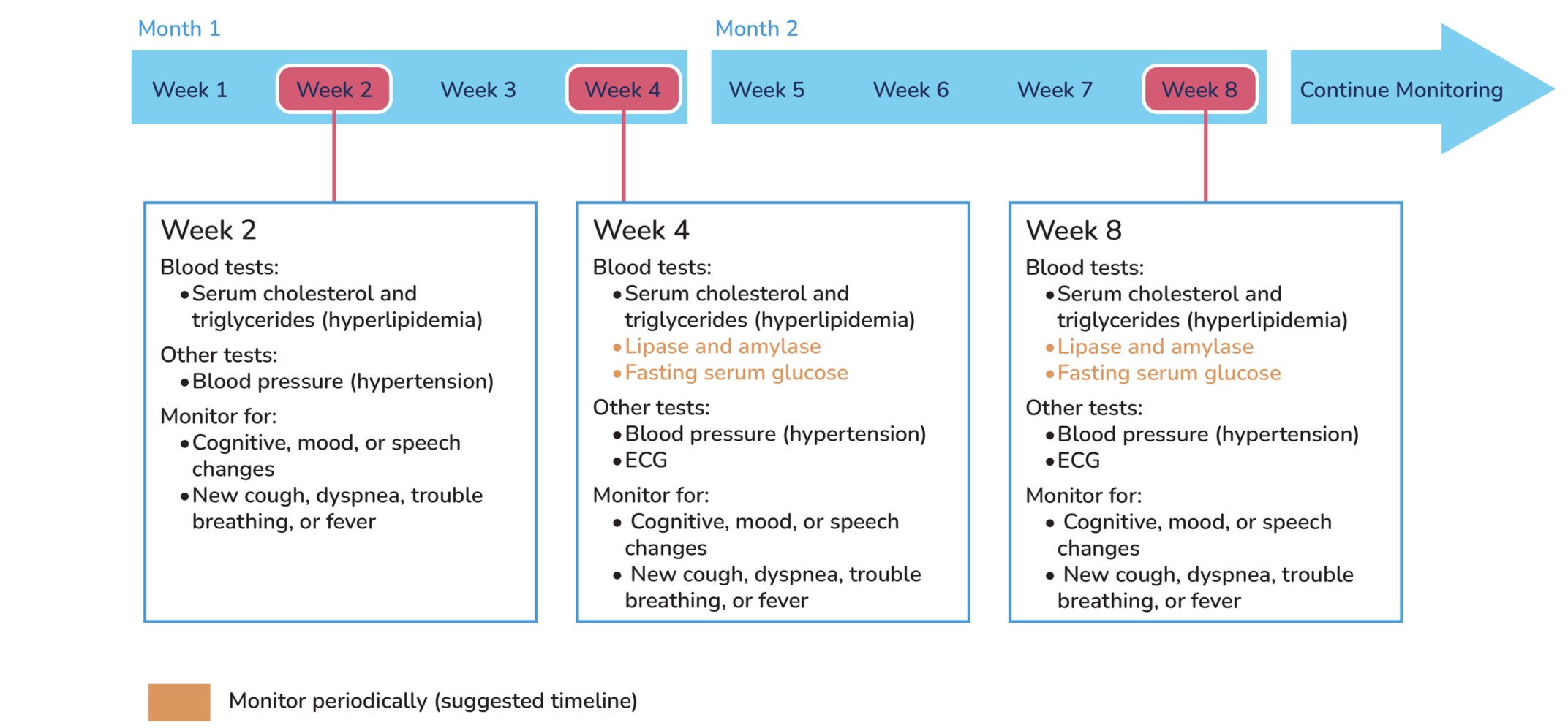
Timeline of Lorlatinib side effects:
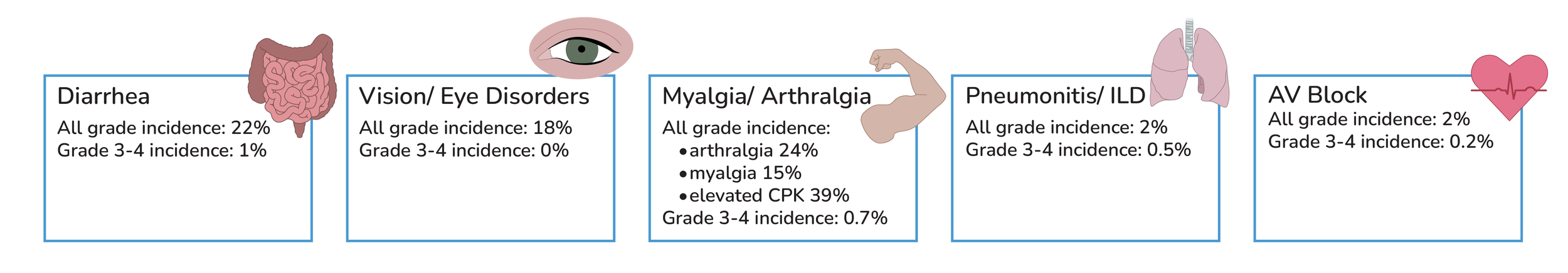
Other Lorlatinib side effects:
Tests to perform before starting treatment:
-
pancreatic enzyme test: elevated lipase and amylase
serum cholesterol and triglyceride levels for hyperlipidemia
fasting serum glucose for hyperglycemia
liver function tests: ALT, AST, and bilirubin levels
-
Evaluate CNS or include neurological exam to establish a baseline.
Lorlatinib can cause CNS effects including:
seizures
psychotic effects
changes in cognitive function
changes in mood (including suicidal ideation)
changes in speech
changes in mental status
changes in sleep
-
Monitor ECG and blood pressure at the start of treatment. Particularly in patients with predisposing conditions to cardiac events.
Advise patients to report symptoms of high blood pressure including:
headaches
dizziness
blurred vision
chest pain
shortness of breath
swelling
-
Establish what a healthy baseline weight for the patient is. This may be a weight prior to cancer diagnosis.
Advise patients to report any rapid or large increases in weight.
Tests to perform during treatment:
General dose guidelines:
-
Recommended starting dose: 100mg orally 1x/day.
Can be taken with or without food (avoid grapefruit products- CYP3A inhibitor, and St. John’s wort- CYP3A inducer).
Medication should be taken at the same time each day.
Tablets should be swallowed whole, do not use if compromised (broken, cracked, etc.).
-
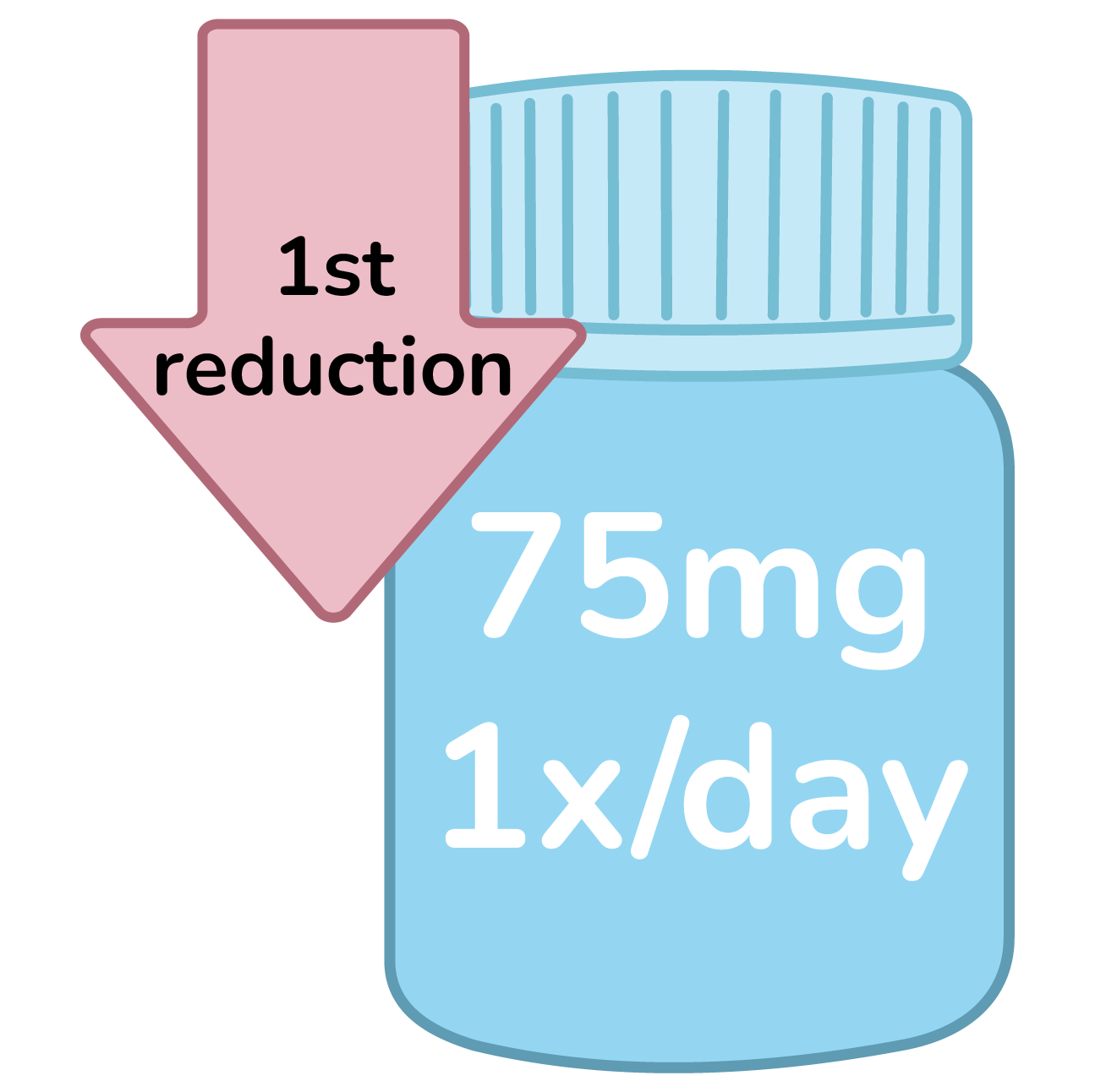
First reduction: 75mg 1x/day
Confirm condition does not fall under specific dose reductions.
-
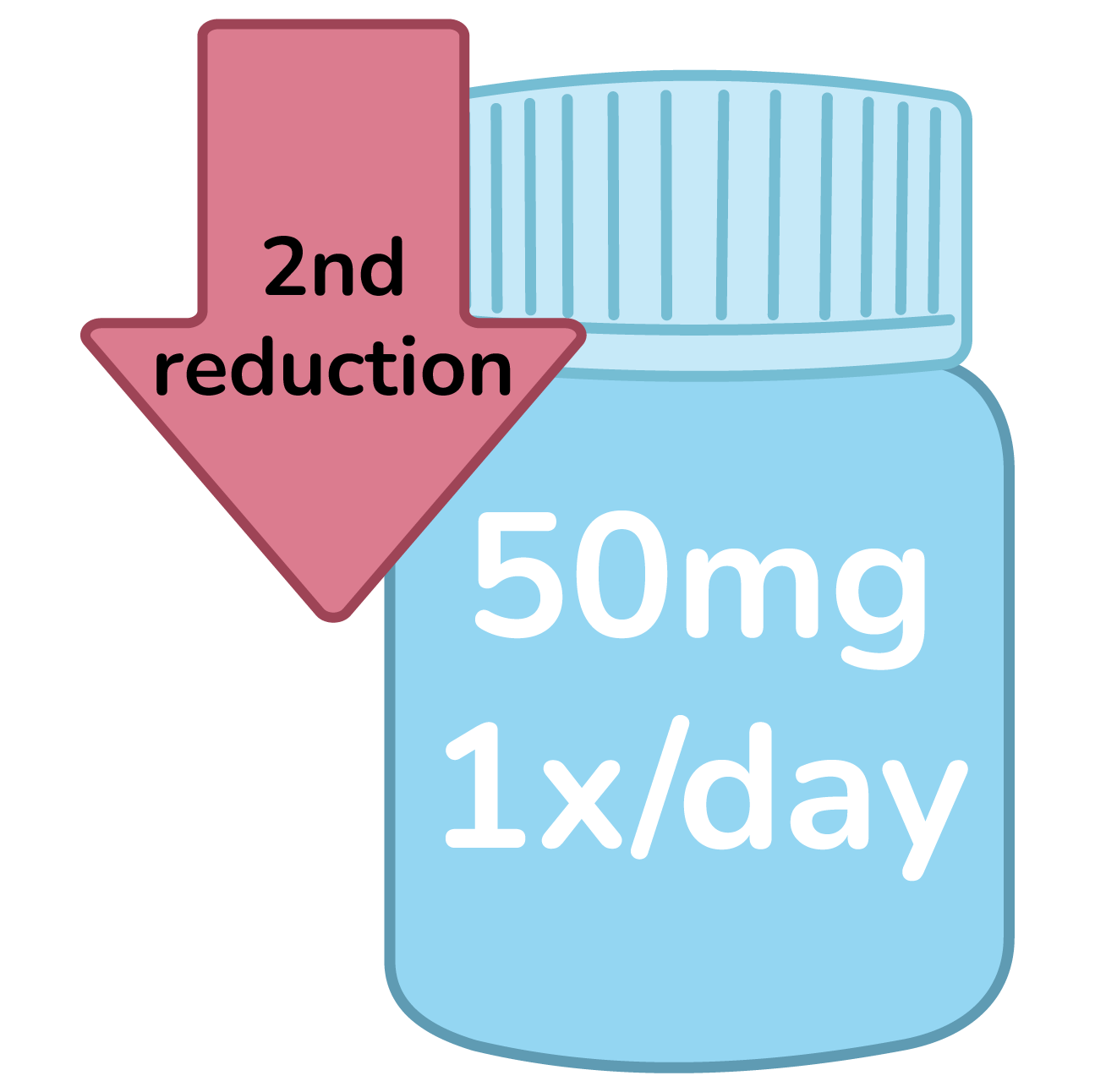
Second reduction: 50mg 1x/day
Confirm condition does not fall under specific dose reductions.
-
Discontinue if patient cannot tolerate 50mg 1x/day.
Specific dose guidelines:
-
Strong CYP3A inhibitors:
Avoid if possible (potential for serious hepatotoxicity).
If unavoidable, reduce to 75mg 1x/day.
If CYP3A inhibitor is discontinued, resume prior dose after a washout period of 3-5 half lives of the CYP3A inhibitor.
Fluconazole:
Avoid if possible.
If unavoidable, reduce starting dose to 75mg 1x/day.
CYP3A inducers:
Discontinue STRONG CYP3A inducers for 3 plasma half-lives before starting lorlatinib.
Avoid use of STRONG CYP3A inducers (reduce lorlatinib plasma concentrations).
If unavoidable, increase starting dose of lorlatinib to 125mg 1x/day.
-
No formal studies have been conducted.
For mild hepatic impairment no dose adjustment is recommended.
For moderate-severe hepatic impairment limited information is available.
-
For mild-moderate impairment no adjustment is recommended.
For severe renal impairments reduce dose to 75mg 1x/day.
Not recommended for patients requiring hemodialysis.
-
If mild to moderate: introduce or modify lipid lowering therapy and continue lorlatinib at same dose.
If severe: introduce or increase the dose of, or change to a new lipid lowering therapy. Withhold lorlatinib until recovery to a mild-moderate level. If severe levels recur, reduce lorlatinib by 1 dose level.
-
If mild: continue at the same dose or withhold dose until recovery to baseline then resume at the same dose or reduce by 1 dose level.
If moderate or severe: withhold dose until toxicity is less than or equal to mild level. Then reduce lorlatinib by 1 dose level.
If life threatening or urgent intervention is indicated permanently discontinue lorlatinib.
-
If ILD or pneumonitis is suspected, withhold dose during investigation.
If ILD or pneumonitis is confirmed, permanently discontinue.
-
If grade 3: withhold lorlatinib until recovered to grade 1 or less.
If grade 3 recurs withhold lorlatinib until recovery to grade 1 or less and reduce dose
If hypertension cannot be managed permanently discontinue
If grade 4: withhold lorlatinib until recovery to grade 1 or less then resume reduced dose or permanently discontinue
If grade 4 recurs permanently discontinue
-
If grade 3 or 4: withhold lorlatinib until hyperglycemia is controlled then resume at reduced dose.
If control isn’t achieved discontinue use.
-
First degree AV block:
If asymptomatic, continue at same dose.
If symptomatic, withhold dose. If symptoms resolve continue at same or reduce 1 dose level.
Second degree AV block:
If asymptomatic, withhold lorlatinib until ECG is checked. If ECG does not show second degree block resume or reduce 1 dose level.
If symptomatic, withhold lorlatinib. If symptoms and second degree block resolve or become asymptomatic first degree AV block, resume or reduce one dose level.
Complete AV block:
Withhold dose.
If pacemaker is placed full dose may be resumed.
If no pacemaker is placed resume at 1 reduced dose level only when symptoms resolve and PR interval is less than 200 msec.
Additional resources:
Dose reductions are common and don’t compromise treatment
People who needed to reduce their dose of lorlatinib early still had similar results to those who did not have their dose reduced early. Both groups lived without their cancer getting worse (including brain metastases), or new tumors spreading to the brain.
Fewer people who took lorlatinib had tumors that spread to the brain, or had brain tumors that got worse compared to those who took crizotinib.
Know what to expect regarding lorlatinib side effects
Side effects of lorlatinib can be managed with dose modification and supportive care. Open communication between healthcare providers and patients is important to educate patients on how to respond to common side effects.
Side effects unique to lorlatinib include high rates of hyperlipidemia and CNS effects.
Lorlatinib has very strong CNS/brain metastases activity
Lorlatinib also has a 2.8% cumulative incidence risk of CNS disease progression at 12 months compared to 9.4% with alectinib.