Crizotinib | Xalkori
Jump to a section
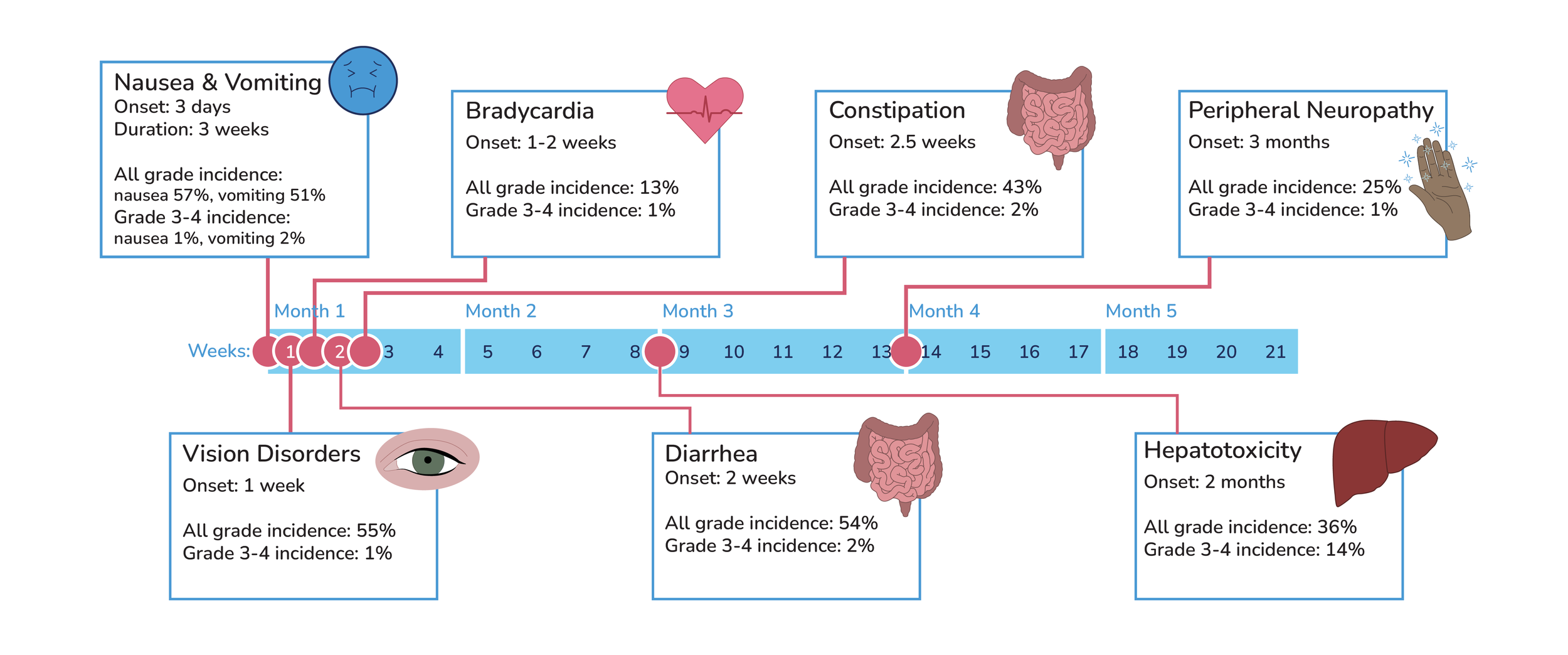
Timeline of Crizotinib side effects:
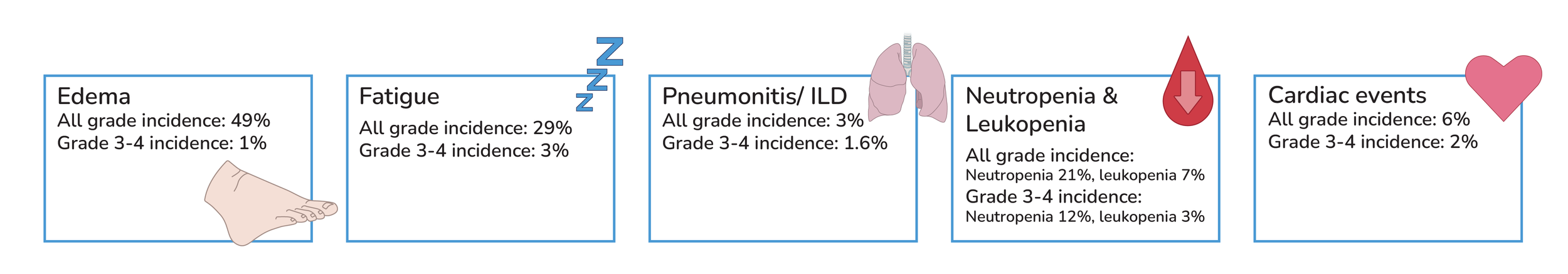
Other Crizotinib side effects:
Tests to perform before starting treatment:
-
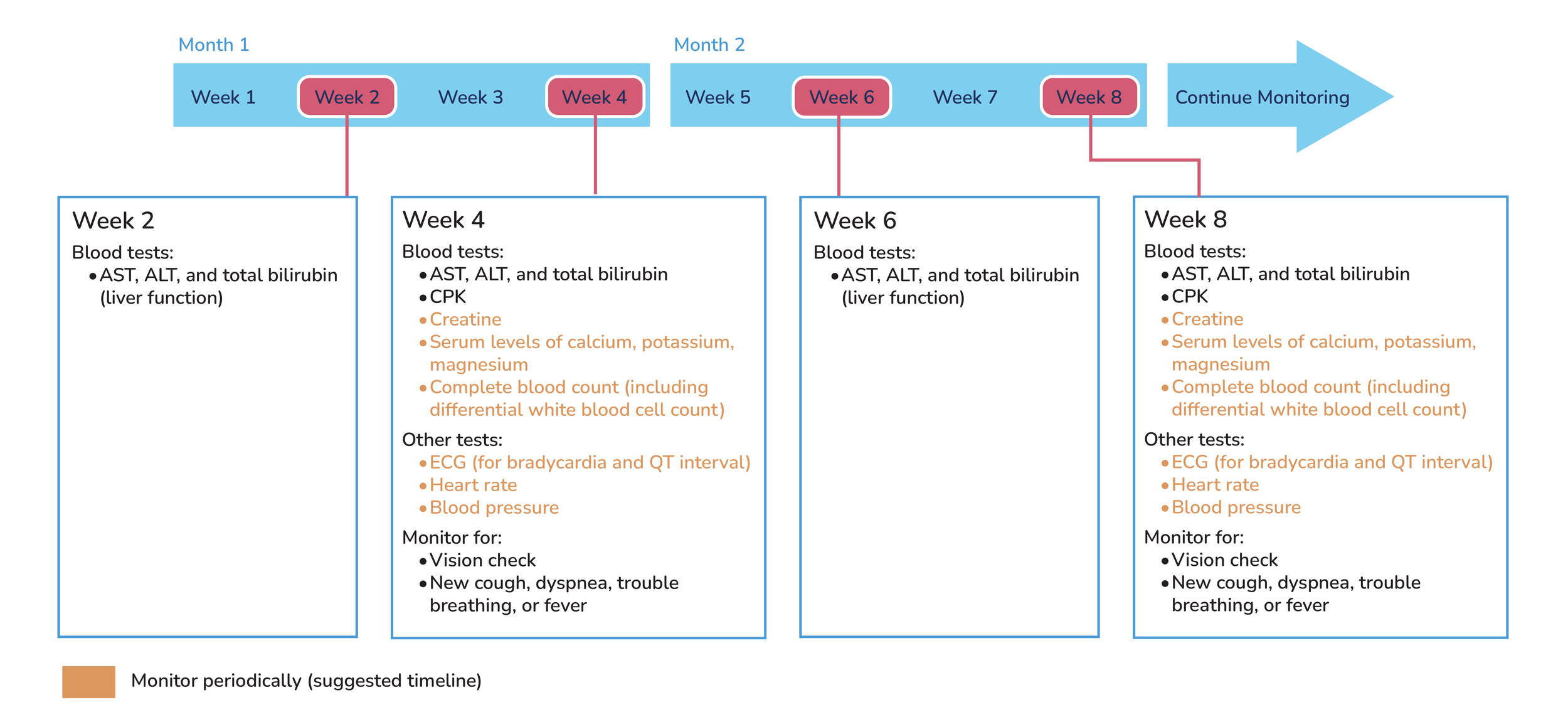
Serum levels of calcium, potassium, and magnesium.
Creatinine levels.
ALT, AST, and bilirubin (liver function test).
Complete blood test with differential wbc count.
-
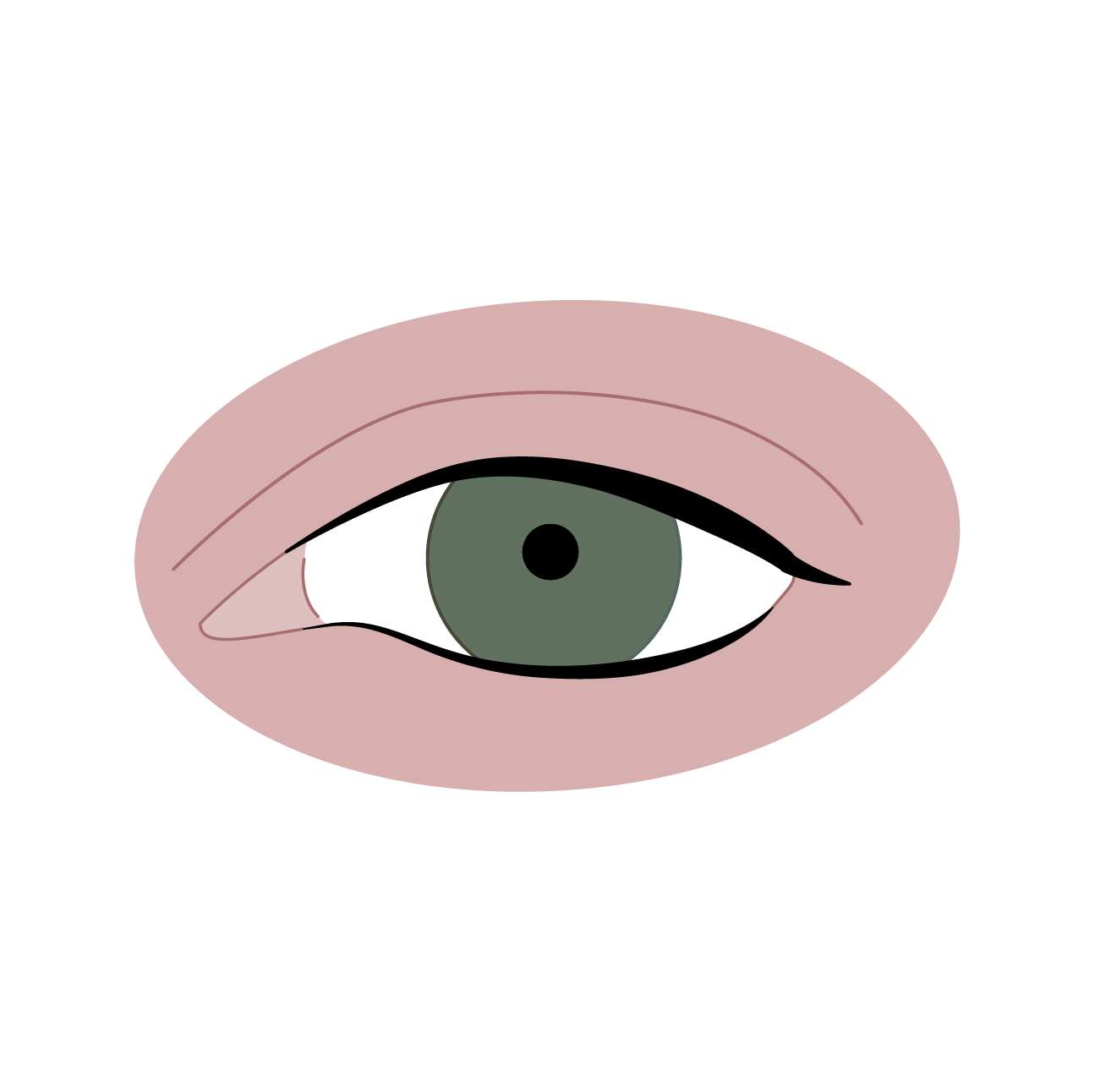
Record vision status.
Vision disorder are common when taking crizotinib. Discuss possible symptoms with patients: most commonly visual impairment, photopsia, blurred vision, and vitreous floaters.
-
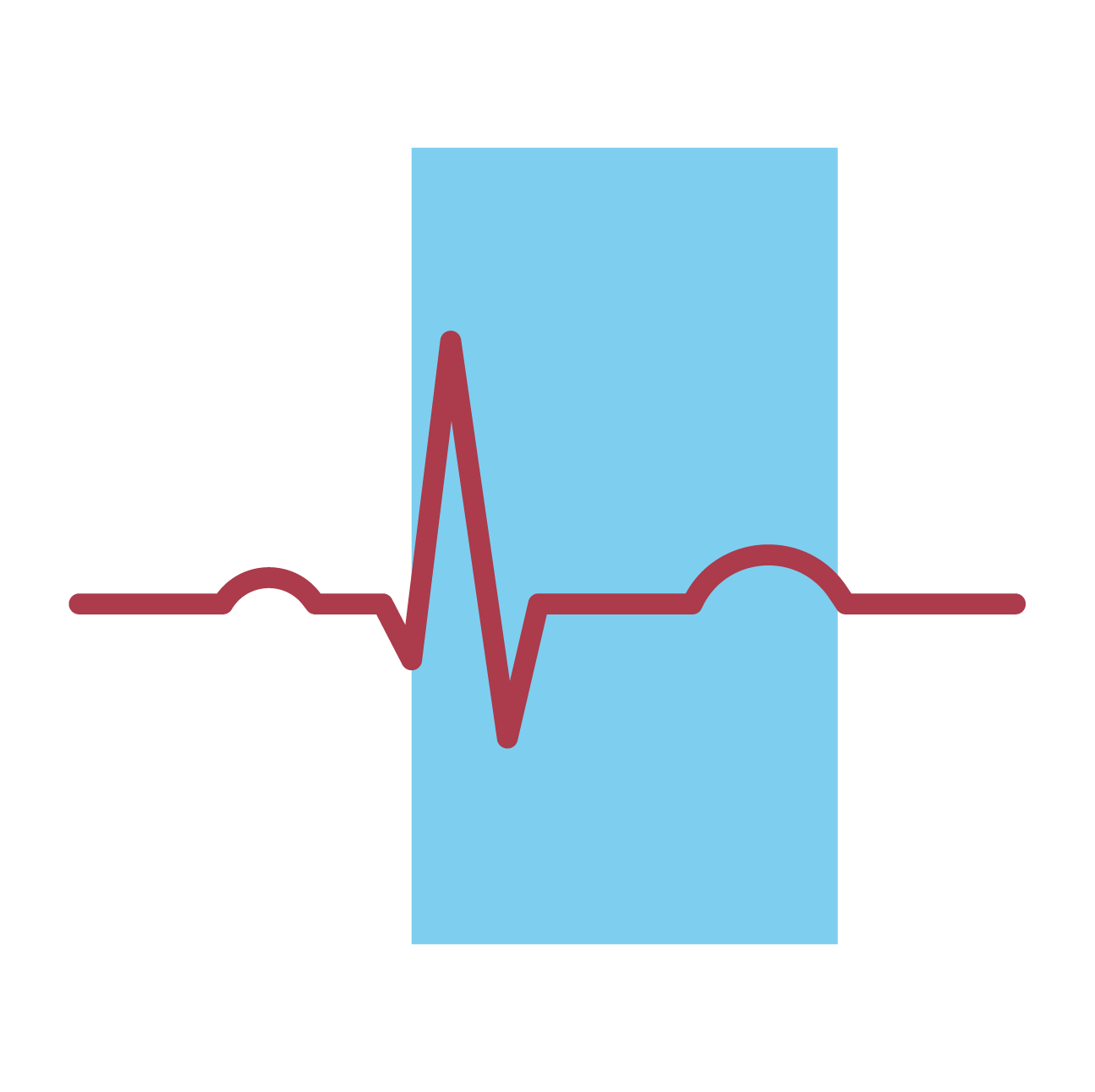
ECG: check for bradycardia and QTc prolongation.
Take baseline heart rate and blood pressure.
Tests to perform during treatment:
General dose guidelines:
-
Recommended starting dose: 250mg 2x/ day
Can be taken with or without food (avoid grapefruit products- CYP3A inhibitor, and St. John’s wort- CYP3A inducer).
Medication should be taken at the same time each day.
Tablets should be swallowed whole, do not use if compromised (broken, cracked, etc.).
-
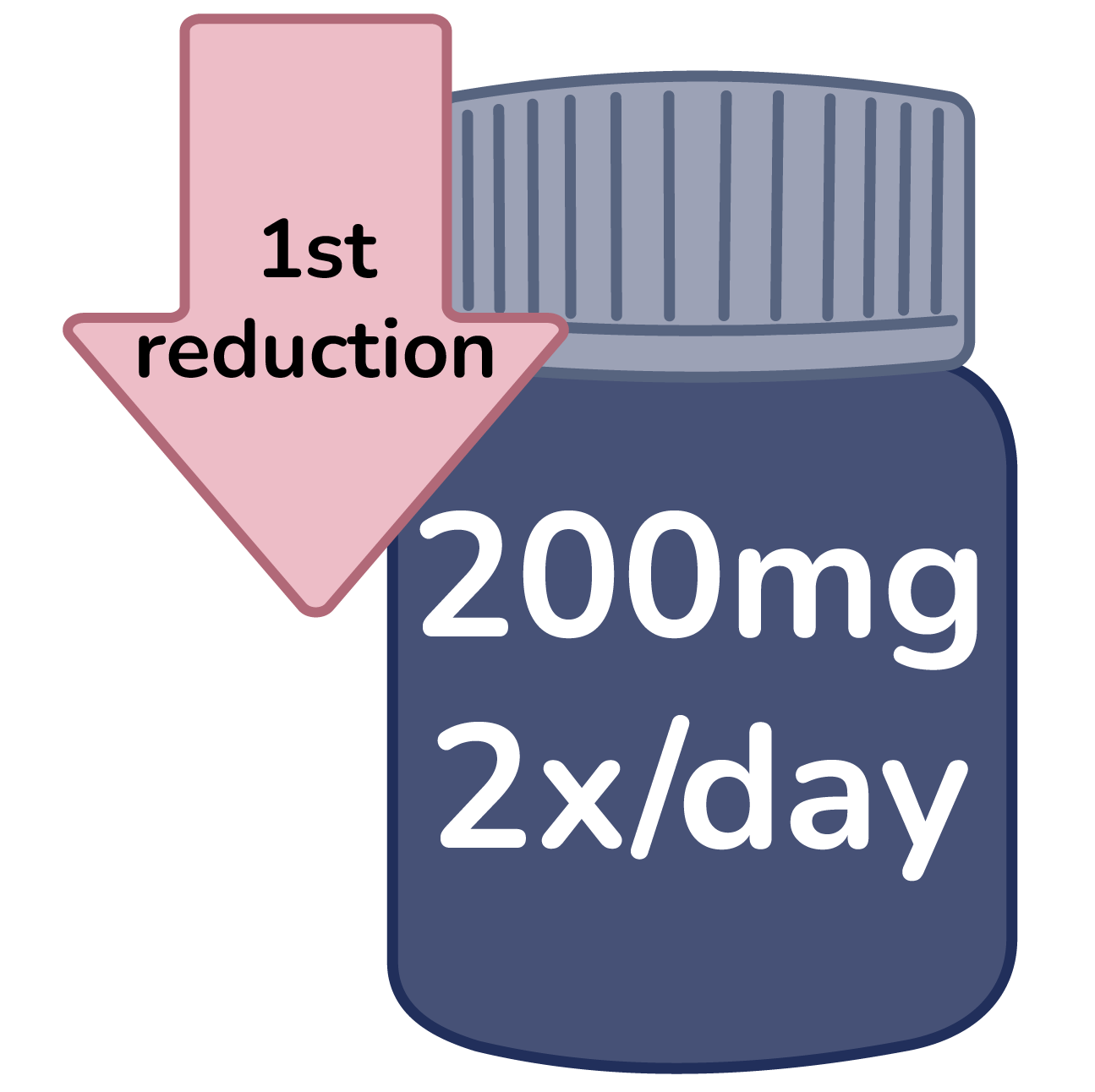
First reduction: 200mg 2x/day
Confirm condition does not fall under specific dose reductions.
-
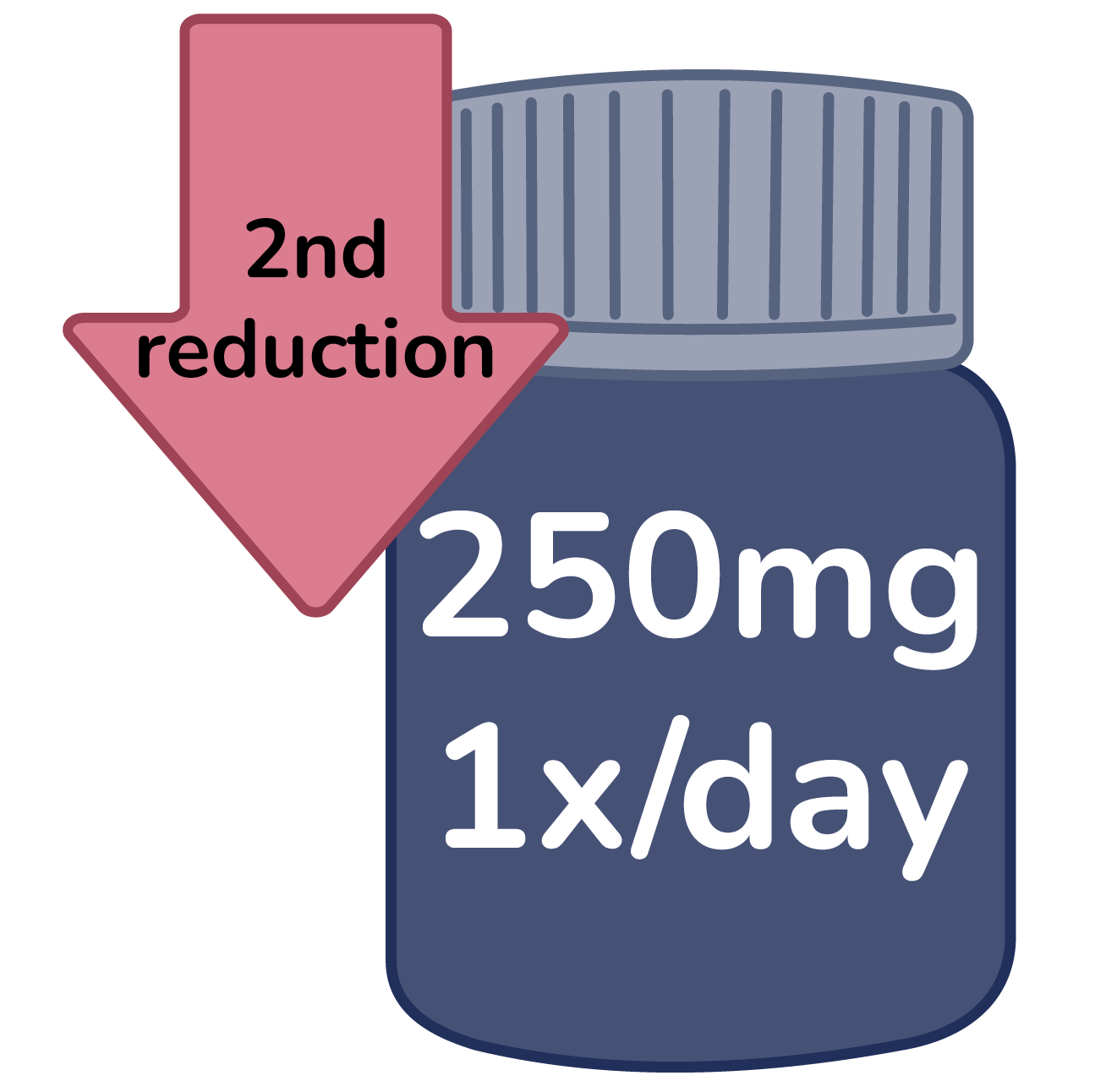
Second reduction: 250mg 1x/day
Confirm condition does not fall under specific dose reductions.
-
Discontinue if patient cannot tolerate 250mg 1x/day.
Specific dose guidelines:
-
Grade 3: withhold until recovered to grade 2 then resume same dose.
Grade 4: withhold until recovered to grade 2 then resume lower dose.
-
Non-hematologic toxicity:
Grade 3 or 4 ALT/AST elevation with less than grade 1 total bilirubin: withhold until recovery then resume at lower dose.
Grade 2/3/4 ALT/AST elevation with grade 2/3/4 total bilirubin: permanently discontinue.
Hepatic impairment”
Moderate impairment: 200mg 2x/day.
Severe impairment: 250mg 1x/ day.
-
Severe not requiring peritoneal dialysis or hemodialysis: 250mg 1x/ day.
-
Grade 2 or 3: withhold until recovery and investigate concomitant medication.
Grade 4: permanently discontinue if no concomitant medication is found.
-
Discontinue during evaluation of severe vision loss.
-
Grade 3: withhold until recovery then resume lower dose.
Grade 4: permanently discontinue.
Additional resources:
Crizotinib significantly prolongs progression-free survival compared to chemotherapy
Crizotinib was the first ALKi developed and was established as a superior treatment to standard first-line chemotherapy in delaying disease progression.
CNS activity with crizotinib
CNS penetration and intracranial efficacy are more limited than later-generation ALK inhibitors.